Home Acid Base CO2 in Ocean Electronegetivity Electron affinity Ionic charge Ionisation energy N2 cycle Metal Extracting Molecular geometry Photographic Chem. Polyatomic ions Practical Ptable Reactivity series Reactions Std Re Potential Strange Reac. Titration
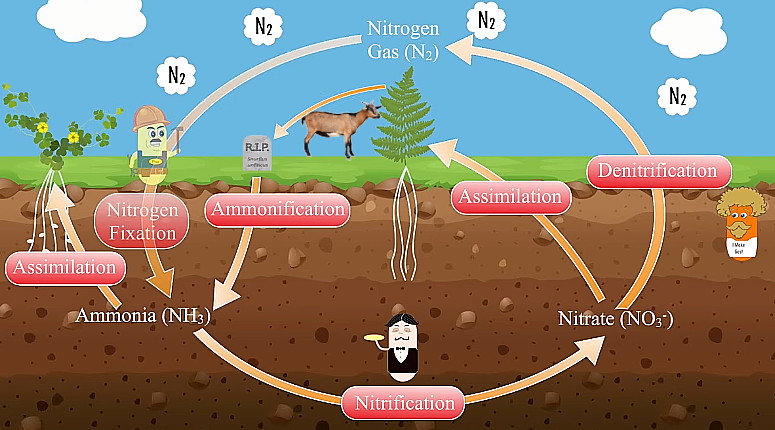
Nitrogen Cycle:
78% of the atmosphere in Nitrogen gas (N2). We breath it in all the time but because of the tripple bond of N atoms
our body cannot break it to use it. Bacteria in soil can break the bond and create Ammonia NH3. This is known as Nitrogen Fixation.
NH3 is used by plants. This process is known as Assimilation. Some bacteria convert Ammonia to Nitrate (NO3-).
This process is called Nitrification. Nitrate is also assimilated by plants. Unused Nitrate is broken down by some bacteria in low
Oxygen environment and release N2 back to the atmosphere. This process is known as Denitrification.
When plants and animals die they decompose and add Ammonia to the soil. This process is called Ammonification.
We receive (essential for life) Nitrogen through our food.