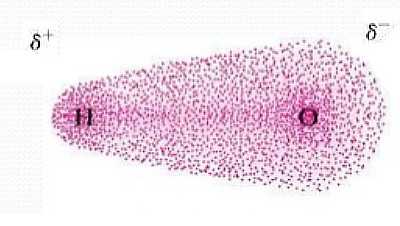
Oxygen is more electro negative then Hydrogen it pulls the shared electron to its side.
Home Acid Base CO2 in Ocean Electronegetivity Electron affinity Ionic charge Ionisation energy N2 cycle Metal Extracting Molecular geometry Photographic Chem. Polyatomic ions Practical Ptable Reactivity series Reactions Std Re Potential Strange Reac. Titration
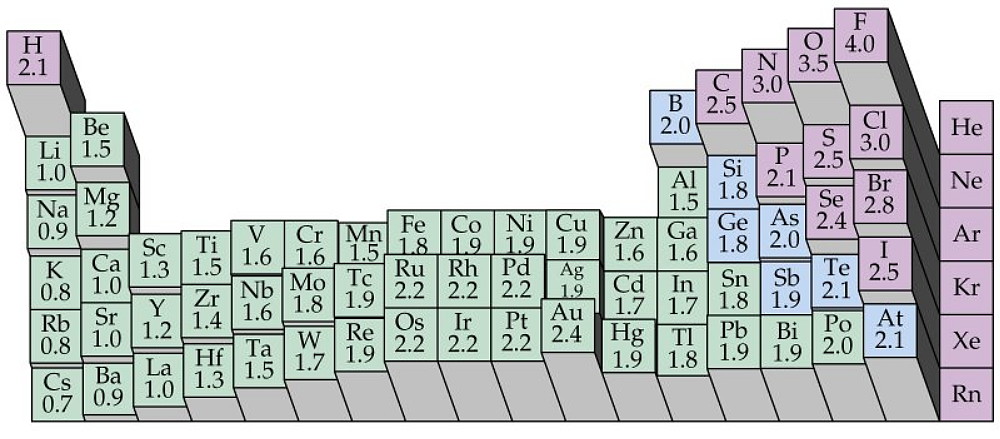
Electronegativity is a measure of an atom`s ability to attract shared electrons to its side.
On the periodic table, electronegativity generally increases as you move from left to right
across a period and decreases as you move down a group.

Oxygen is more electro negative then Hydrogen it pulls the shared electron to its side.