Home Acid Base CO2 in Ocean Electronegetivity Electron affinity Ionic charge Ionisation energy N2 cycle Metal Extracting Molecular geometry Photographic Chem. Polyatomic ions Practical Ptable Reactivity series Reactions Std Re Potential Strange Reac. Titration
Ocean-Atmosphere Exchange:
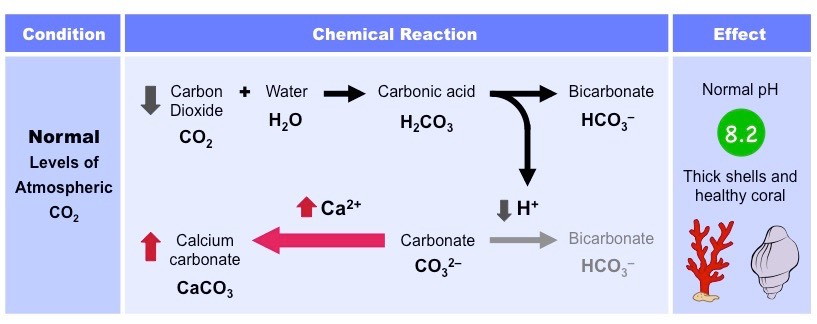
Typically, when carbon dioxide is dissolved in the ocean, it combines with water molecules to form carbonic acid (H2CO3)
The carbonic acid then dissociates to form bicarbonate ions (HCO3-) and hydrogen ions (H+)
The ocean also contains carbonate ions (CO3-), which are absorbed by coral and molluscs to form calcium carbonate (CaCO3)
Calcium carbonate is used to form the hard exoskeletons of reef-building corals and is used by molluscs to develop shells
H+ ions can reduce the stock of carbonate ions in the ocean by combining with it to form bicarbonate
Hence, the levels of H+ ions must be kept low to ensure that their is sufficient stock of carbonate ions for aquatic organisms
Ocean Acidification
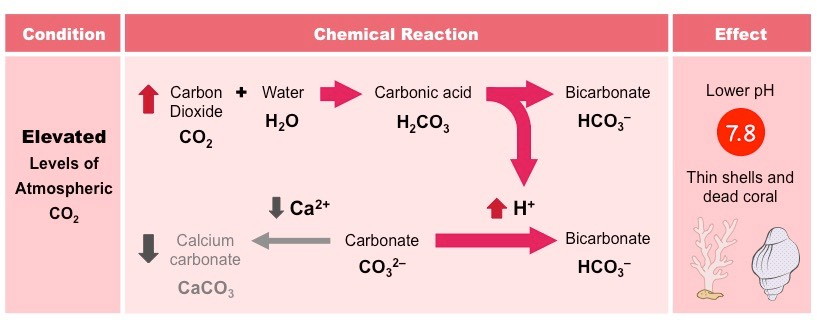
As a result of deforestation and the increased burning of fossil fuels, atmospheric carbon dioxide concentrations have increased
With more CO2 being absorbed by the oceans, there is an associated increase in the production of H+ ions
These H+ ions lower the pH of the ocean, causing acidification (ocean pH has dropped ~0.2 since the industrial revolution)
The H+ ions will also combine with carbonate ions, reducing the amounts available to marine organisms
This will result in the formation of thinner, deformed shells and reduce the population numbers of reef-building corals
The reduction in pH will also dissolve calcium carbonate structures, enhancing the damage to shells and corals