 | Bloomery furnace. Produces wrought iron. |
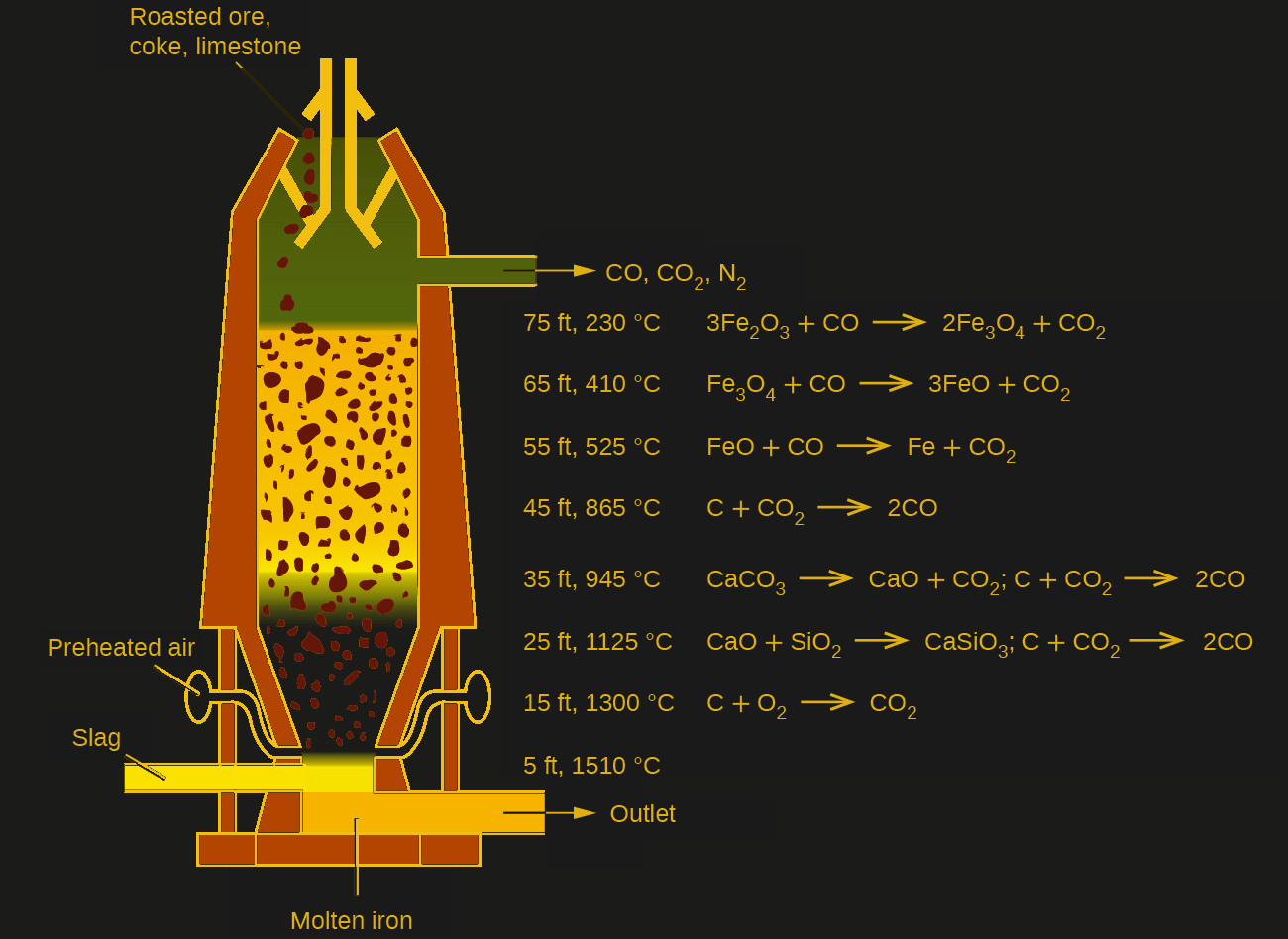 | Modern Blast Furnace. |
 | Iron(II) oxide or ferrous oxide (FeO)
Ore: Wustite |
 | Iron(III) oxide or ferric oxide (Fe2O3)
Ore: Hematite Making Fe2O3 in lab : Video |
 | Iron oxide pigments |
Home Acid Base CO2 in Ocean Electronegetivity Electron affinity Ionic charge Ionisation energy N2 cycle Metal Extracting Molecular geometry Photographic Chem. Polyatomic ions Practical Ptable Reactivity series Reactions Std Re Potential Strange Reac. Titration
| H (o-o) Hydride H- | He | ||||||||||||||||
| Li + | Be 2+ Beryl | (o-o) Diatomic | B 3+, 3- Borax | C | N (o-o) Nitride 3- | O (o-o) Oxide 2- | F (o-o) Fluoride 1- | Ne | |||||||||
| Na + | Mg 2+ Dolomite | Al 3+ Bauxite | Si 4+ Quartz | P Phosphide 3- | S Sulfide 2- | Cl (o-o) Chloride 1- | Ar | ||||||||||
| K + Potash | Ca 2+ Limestone | Sc +++ | Ti 4+ | V 3+, 4+, 5+ | Cr 2+, 3+ | Mn 2+, 3+ | Fe 2+, 3+ Bog ore | Co 2+, 3+ | Ni 2+,3+ | Cu +, 2+ Malachite | Zn ++ | Ga 3+ | Ge | As 3-, 3+, 5+ | Se Selenide 2- | Br (o-o) Bromide 1- | Kr |
| Rb + | Sr 2+ | Y +++ | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag + | Cd ++ | In 3+ | Sn 2+, 4+ Cassiterite | Sb | Te 2-, 2+, 4+, 6+ | I (o-o) Iodide 1- | Xe |
| Cs + | Ba 2+ | Hf | Ta | W | Re | Os | Ir | Pt | Au +, 3+ | Hg 2+ | Tl | Pb 2+, 4+ Cerussite | Bi 3+ | Po | At | Rn* | |
| Fr* | Ra* | Rf* | Db* | Sg* | Bh* | Hs* | Mt* | Ds* | Rg* | Cn* | Nh* | Fl* | Mc* | Lv* | Ts* | Og* |
Iron (Fe) | Iron atom | Iron videos |
|